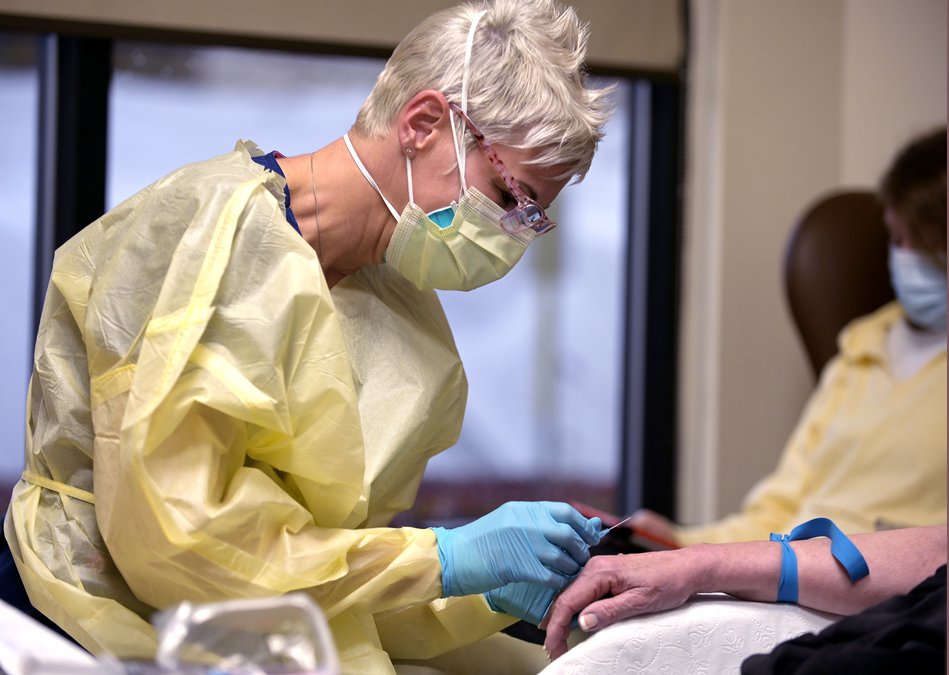
Health care providers may have their supply of monoclonal antibodies disrupted as the federal government has changed the way the highly effective COVID-19 treatment will be distributed across the country.
Federal changes coming to distribution of this COVID treatment